Class 10 Science Chapter 5 Revision Notes Periodic Classification of Elements
Class 10 Science Chapter 5 Revision Notes Periodic Classification of Elements, (Science) exams are Students are taught thru NCERT books in some of the state board and CBSE Schools. As the chapter involves an end, there is an exercise provided to assist students to prepare for evaluation. Students need to clear up those exercises very well because the questions inside the very last asked from those.
Sometimes, students get stuck inside the exercises and are not able to clear up all of the questions. To assist students, solve all of the questions, and maintain their studies without a doubt, we have provided a step-by-step NCERT Revision Notes for the students for all classes. These answers will similarly help students in scoring better marks with the assist of properly illustrated Notes as a way to similarly assist the students and answer the questions right.
Class 10 Science Chapter 5 Revision Notes Periodic Classification of Elements
Periodic Laws and their Limitations
- Need for Periodic Classification: To make the study of elements easy, elements have been divided into few groups in such a way that elements in the same group have similar properties. Now, study of a large number of elements is reduced to a few groups of elements.
- Dobereiner’s Triads: When elements are arranged in the order of increasing atomic masses, groups of three elements (known as triads), having similar chemical properties are obtained.
- The atomic mass of the middle element of the triad was roughly the average of the atomic masses of the other two elements.
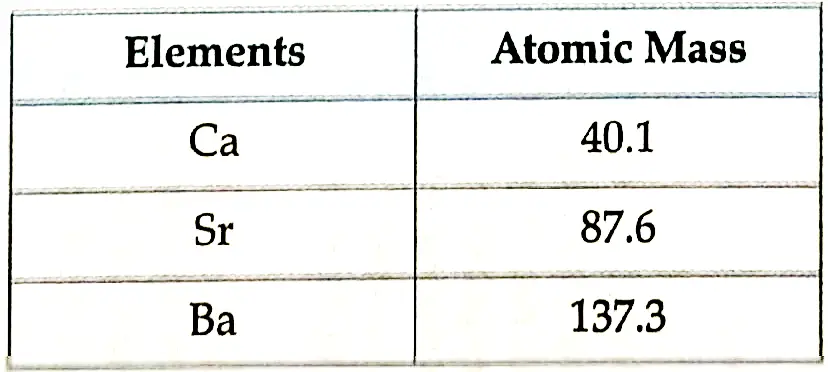
- Limitation: Dobereiner could identify only three triads. He was not able to prepare triads of all the known elements.
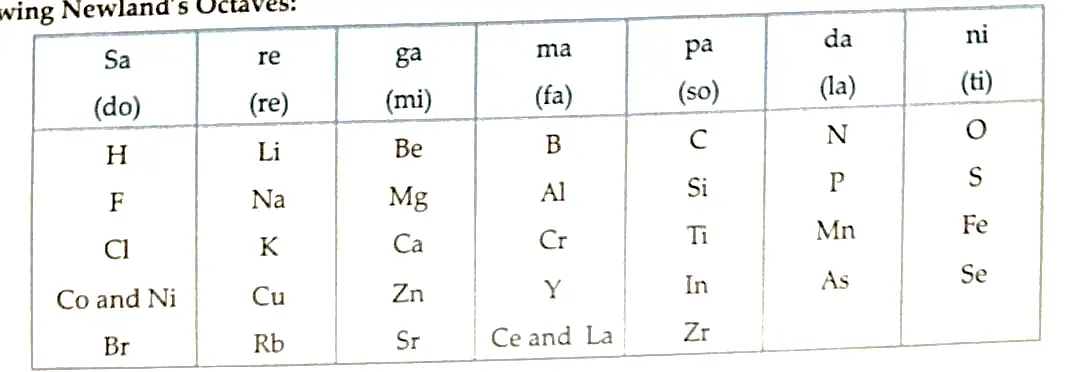
- Newland’s Law of Octaves: John Newlands arranged the elements in the order of increasing atomic masses. It states that when the elements are arranged in increasing order of atomic masses, the properties of the eighth element are a kind of repetition of the first, just like notes of music.
Table showing Newland’s Octaves:
-
Limitations of Newland’s law of octaves:
- The law was applicable to elements up to calcium (Ca) only.
- It contained only 56 elements. Furthert, it was assumed by Newlands that only 56 elements existed in nature and no more elements would be discovered in the future.
- In order to fit elements into the table, Newland adjusted two elements in the same column as fluorine, chlorine and bromine which have very different properties than these elements. Iron, which resembles cobalt and nickel in properties, has been placed differently away from these elements
Mendeleev’s Periodic Table:
Dmitri Ivanovich Mendeleev, a Russian chemist, was the most important contributor to the early development of a periodic table of elements as in this the elements were arranged on the basis of their atomic mass and chemical properties
Characteristics of Mendeleev’s Periodic Table:
- Mendeleev arranged all the 63 known elements in increasing order of their atomic masses.
- The table consists of vertical columns called Groups’ and horizontal rows called Periods’.
- The elements with similar physical and chemical properties came under same groups.
Mendeleev’s Periodic Law:
- The properties of elements are the periodic functions of their atomic masses.
Merits of Mendeleev’s Periodic Table:
- Mendeleev left some blank spaces for undiscovered elements.
- Mendeleev predicted the discovery of some elements and named them as eka boron, eka aluminium and eka silicon.
- Noble gases discovered later could be placed without disturbing the existing order.
Limitations of Mendeleev’s periodic table:
- Position of Hydrogen: He could not assign a correct position to hydrogen as hydrogen resembles alkali metals as well as halogens.
- Position of Isotopes: Isotopes are placed in same position though they have different atomic masses.
- Separation of chemically similar elements while dissimilar elements are placed in the same group.
Modern Periodic Table:
- Henry Moseley gave a new property of elements, ‘atomic numbers’ and this was adopted as the basis of Modern Periodic Table.
Modern Periodic Law:
- Properties of elements are the periodic functions of their atonic numbers.
Periodic Elements and Periodic Properties
Position of elements in modern periodic table:
- The Modern Periodic Table consists of 18 groups and 7 periods.
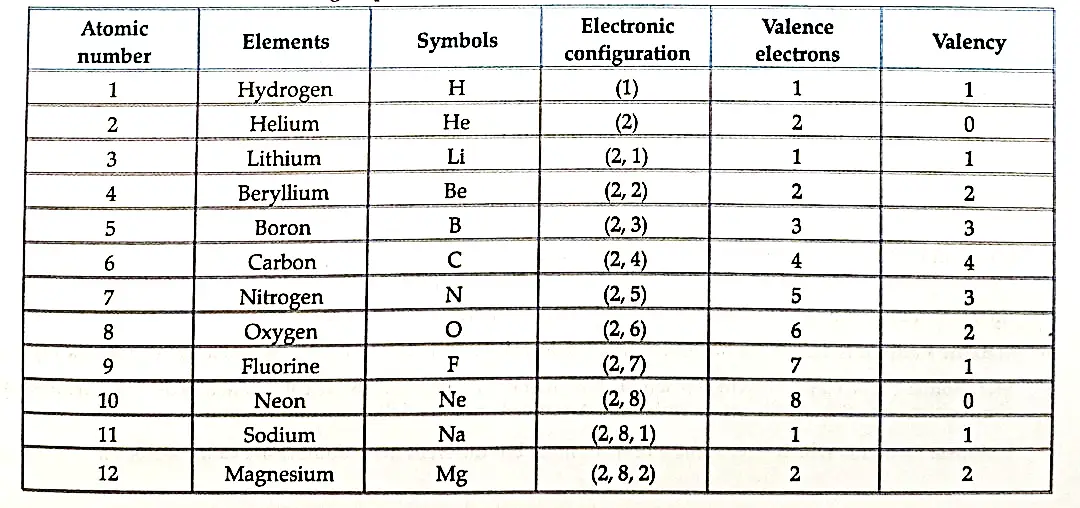
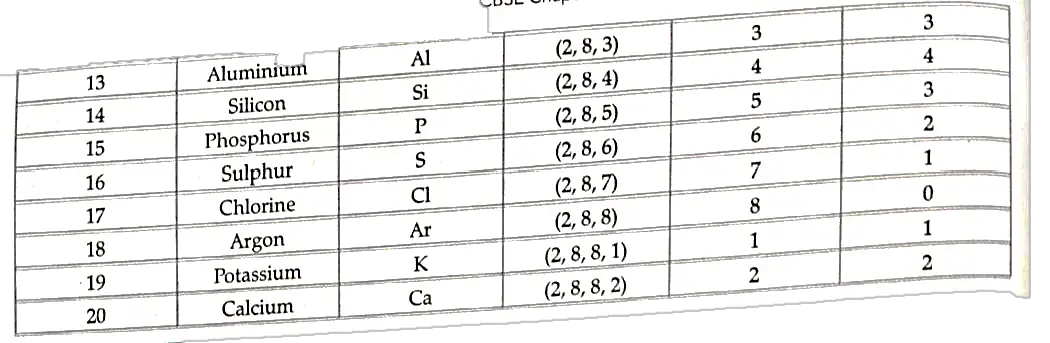
- Elements present in any one group have the same number of valence electrons, Also. the number of shells increases as we go down the group.
- Elements present in any one period, contain the same number of shells.
- Also, with increase in atomic number by one unit on moving from left to right, the valence shell electron increases by one unit.
- Each period marks a new electronic shell getting filled.
Trends in the Modern Periodic Table:
( i ) Periodicity in Properties:
- The properties of elements depend upon the electronic configuration which changes along a period and down a group in the periodic table.
- The periodicity properties ie., repetition of properties after a regular interval is due to similarity in electronic configuration.
( ii ) Tendency to lose or gain electron:
- Chemical reactivity of an element depends upon the ability of its atoms to donate or accept electrons.
( iii ) Variations of tendency to lose electron down the group:
- Tendency to lose electron goes on increasing down the group.
Reason: It is due to the increase in the distance between the valence electrons and the nucleus as the atomic size increases down the group, the force of attraction between the nucleus and the valence electrons decreases, therefore, tendency to lose electron also increases down the group.
( iv ) Variation of tendency to lose electron along a period:
- It goes on decreasing generally along a period from left to right with decrease in atomic size.
Reason: Due to decrease in the atomic size, the force of attraction between the valence electrons and the nucleus increases and therefore, electrons cannot be removed easily.
( v ) Variation of tendency to gain electron down the group:
- It goes on decreasing down the group in general.
Reason: Due to increase in atomic size, the force of attraction between the nucleus and the electron to be added becomes less.
( vi ) Variation of tendency to gain electron along a period:
- It increases left to right in a period.
Reason: It is due to decrease in the atomic size which leads to an increase in the force of attraction between the nucleus and the electron to be added.
Metallic and non-metallic character: Group 1 to 12 are metals. Group 13 to 18 comprises non-metals, metalloids, and metals.
Properties of Metals:
- They are malleable.
- They are ductile.
- They are good conductors of heat and electricity.
- They have generally 1 to 3 valence electrons.
- They have the same or less number of electrons in their outermost shell than the number of shells.
- They are mostly solids.
Properties of Non-metals:
- They exist in solid, liquid or gaseous state.
- Non-metals are generally brittle.
- They are non-conductors.
- They have 4 to 8 valence electrons.
Atomic radii increase down the group.